The FDA has said that the controversial drug Avastin should be phased out as a treatment for metastatic breast cancer. Recent studies show that its benefits are outweighed by dangerous side effects.
The FDA has said that the controversial drug Avastin should be phased out as a treatment for metastatic breast cancer. Recent studies show that its benefits are outweighed by dangerous side effects.
The announcement does not affect Avastin's status as a drug that can be prescribed for lung cancer, kidney cancer, colorectal cancer and brain cancer.
"Along with those disappointing findings, serious side effects became apparent in patients taking Avastin, including high blood pressure, internal bleeding, perforated internal organs, heart failure and heart attacks, and in some cases, even swelling of the brain."

 Health Glance
Health Glance There's good news on the fluoride front in New York City this week, but your help is needed there (and also in San Diego). Councilor Peter Vallone is introducing a bill to the New York City Council that would end fluoridation in NYC. We need help from all our New York City readers to support this effort to protect the safety of the NYC water supply and remove this toxic chemical from the tap water there.
There's good news on the fluoride front in New York City this week, but your help is needed there (and also in San Diego). Councilor Peter Vallone is introducing a bill to the New York City Council that would end fluoridation in NYC. We need help from all our New York City readers to support this effort to protect the safety of the NYC water supply and remove this toxic chemical from the tap water there. The doctor who linked childhood autism to a vaccine and has been branded a fraud by the British Medical Journal (BMJ) said he was the victim of a smear campaign by drug manufacturers.
The doctor who linked childhood autism to a vaccine and has been branded a fraud by the British Medical Journal (BMJ) said he was the victim of a smear campaign by drug manufacturers. A second person denied transplant coverage by Arizona under a state budget cut has died, with this death "most likely" resulting from the coverage reduction, a hospital spokeswoman said Wednesday.
A second person denied transplant coverage by Arizona under a state budget cut has died, with this death "most likely" resulting from the coverage reduction, a hospital spokeswoman said Wednesday. The Obama administration, reversing course, will revise a Medicare regulation to delete references to end-of-life planning as part of the annual physical examinations covered under the new health care law, administration officials said Tuesday.
The Obama administration, reversing course, will revise a Medicare regulation to delete references to end-of-life planning as part of the annual physical examinations covered under the new health care law, administration officials said Tuesday.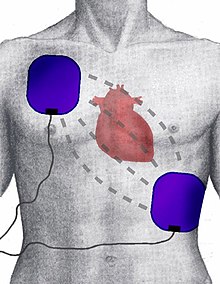 Doctors are implanting high-tech heart devices in thousands of people who probably do not need them, a new study finds. The procedures cost more than $35,000, involve surgery and anesthesia, and may unnecessarily harm some patients.
Doctors are implanting high-tech heart devices in thousands of people who probably do not need them, a new study finds. The procedures cost more than $35,000, involve surgery and anesthesia, and may unnecessarily harm some patients. Activating dormant stem cells in the scalp could be the key to banishing baldness, scientists believe. Researchers made the discovery after examining tissue sample from men undergoing hair transplants.
Activating dormant stem cells in the scalp could be the key to banishing baldness, scientists believe. Researchers made the discovery after examining tissue sample from men undergoing hair transplants.